November 6, 2022
Happy to share that I’ve moved to my new position. I’ve joined USDA-APHIS, Biotechnology Regulatory Services (BRS), Biotechnology Risk Analysis Programs (BRAP), Plant Evaluation Branch, as a Biological Scientist. I’ll be working on regulatory status documents related to various genetically modified organisms.
June 10, 2022
Very happy to see that our new paper on meta-barcoding of the spotted lanternfly gut contents is published today in journal Insects, the Special Issue “Advances on Invasive Insect Pests: Insect Behavior, Host Plant Usage, Biocontrol, and More” which I co-edit!
Our study investigated host-plant usage by the first nymphal instars, which are more challenging to find and monitor than other stages. Using DNA metabarcoding of nymphal gut contents (i.e., detection of DNA from multiple ingested plants) in this study we determined and characterized the ingested plants that could be included in a broad host-plant range of early nymphal stages of L. delicatula. This, in turn, have important applications for developing effective management programs to control this invasive pest.
Here is our paper:
McPherson, C., Avanesyan, A., and W.O. Lamp. (2022) Diverse host plants of the first instars of the invasive Lycorma delicatula: insights from eDNA metabarcoding. Insects: Special Issue”Advances on Invasive Insect Pests: Insect Behavior, Host Plant Usage, Biocontrol, and More”, 13(6), 534.
May 25, 2022
Developed course DNA BARCODING FOR EVERYONE
Happy to share the new course I developed, “DNA barcoding for Everyone”! This course includes all the materials and protocols for the DNA barcoding workflow which I developed in the Lamp Lab for our research projects. The course outline with video tutorials and protocols are available here.
December 25, 2021
Our paper on responses of Miscanthus sinensis cultivars to herbivory is published!
I’m happy to share that our paper entitled “Response of five Miscanthus sinensis cultivars to grasshopper herbivory: implications for monitoring of invasive grasses in protected Areas” is published in Plants, Special Issue “Invasive Alien Species in Protected Areas”.
In this study, we conducted field and greenhouse experiments to explore plant resistance and tolerance to grasshopper herbivory in five cultivars of Miscanthus sinensis. The results of this project demonstrated that plant responses varied among the cultivars during a season; all the cultivars, but “Zebrinus”, demonstrated a significant increase in plant tolerance by the end of the growing season regardless of the amount of sustained leaf damage. Different patterns in plant responses from “solid green” and “striped/spotted” varieties were recorded, with the lowest plant resistance detected for “Autumn Anthem” in the cage experiment. Our results have important applications for monitoring low-risk invaders in protected areas, as well as for biotic resistance of native communities to invasive grasses.

May 6, 2021
I’m happy to share my little (so far!) collection of my nature drawings with a heavy focus on insect drawings, of course! I always loved to draw but last year I started drawing insects. I love it! At first, I’ve decided to draw my favorite study species only, but it is hard to resist drawing anything beautiful I see. So my collection extended to some other insect species and plants. Last January, I took an online course “Drawing Nature, Science and Culture: Natural History Illustration 101”, a course of study offered by NewcastleX, an online learning initiative of The University of Newcastle. I proudly completed all the assignments (some of them are below) and earned a certificate. The drawing of the eastern gray squarel was my final exam drawing.
Here is my page at deviantart.com where I post my new drawings as I finish them. This and last week two of my drawings, the spotted lanternfly and the silver maple, were featured at ThePencilClub - group. So exciting!















April 3, 2021
Our systematic review on PCR approaches to insect gut content analysis is published!
I’m happy to share that our review paper entitled “Choosing an effective PCR-based approach for diet analysis of insect herbivores: A systematic review.” is published in Journal of Economic Entomology. In this study we reviewed the commonly used PCR-based approaches in studies published from 1977 to 2019, to provide researchers with the information on the tools which have been shown to be effective for obtaining and identifying ingested plants.
In our systematic review, we were specifically focused on the following questions: (1) What insect groups were used for detecting ingested plant species using a PCR-based approach? (2) Which DNA extraction methods have been used for detecting plant DNA from insect guts? (3) Which DNA regions have been shown to allow for successful identification of plant species? (4) How is the presence of the targeted plant DNA fragment verified?
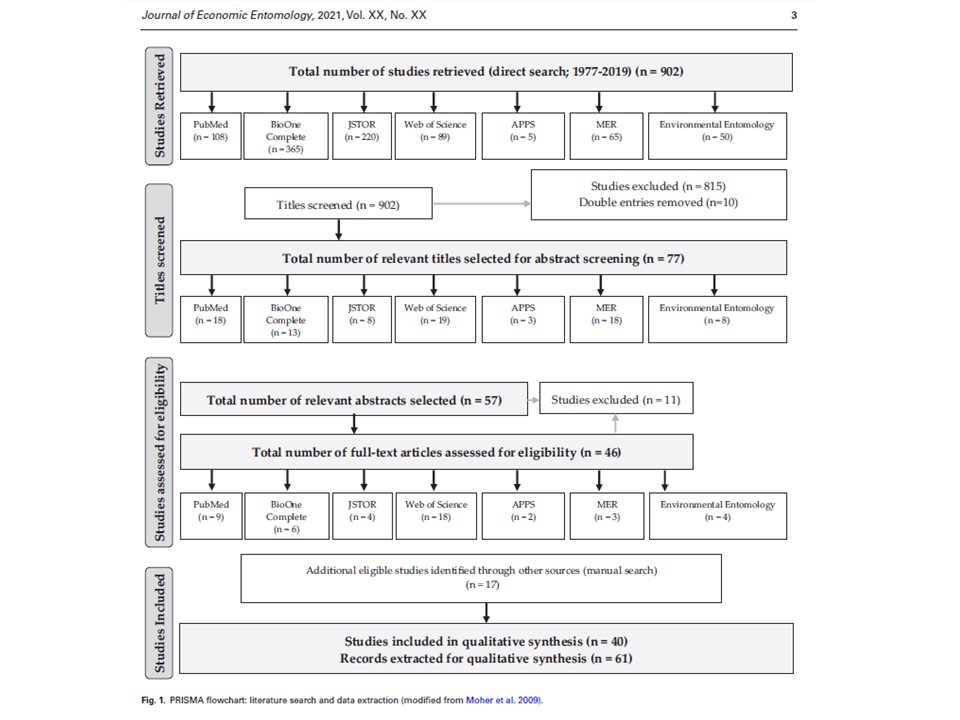
Our results showed that among five insect orders used in the retrieved studies Coleoptera and Hemiptera were prevalent (33 and 28% of all the records, respectively). In 79% of the studies a DNA barcoding approach was employed. In a substantial number of studies Qiagen DNA extraction kits and CTAB protocol were used (43 and 23%, respectively). Of all records, 65% used a single locus as a targeted plant DNA fragment; trnL, rbcL, and ITS regions were the most frequently used loci. Sequencing was the dominant type of among DNA verification approaches (70% of all records). This review provides important information on the availability of successfully used PCR-based approaches to identify ingested plant DNA in insect guts, and suggests potential directions for future studies on plant–insect trophic interactions.
Here is our paper.
February 5, 2021
Will serve on the University Senate!
I’m very grateful and I feel deeply honored to be elected as a PTK senator, to represent the needs, interests, and views of professional track faculty on the University Senate. This is my first experience with this, and I’m really looking forward to serving on the Senate and continuing to contribute to the academic life of our university as much as I can.
January 1, 2021
Reviewer Board Member for Agriculture!
I was invited to serve on Reviewer Board Member for Agriculture. I really enjoy serving as a reviewer and find it very rewarding. As an author, I got to appreciate the time the editors and reviewers dedicate and effort they make during the peer-review process, as well as the challenges along the way. Being an reviewer means for me not only “returning” the time and efforts to the other reviewers, but it is also a non-stop learning process through which I improve my research and share what I learn with other researchers.
November 4, 2020
Our paper on host plant DNA detection from the potato leafhopper gut contents is published!
I’m happy to share that our paper entitled “Detecting Ingested Host Plant DNA in Potato Leafhopper (Hemiptera: Cicadellidae): Potential Use of Molecular Markers for Gut Content Analysis” is published in Journal of Economic Entomology. In this study we focused on optimizing a DNA-based method for host plant identification of E. fabae and investigating the longevity of the ingested plant DNA as one of the potential applications of the protocol.
Here is our paper.
July 27, 2020
Presented at Botany 2020 - Virtual!
This week I presented at the Botany-2020 virtual meeting, Annual Meeting of the Botanical Society of America. It was quite a new experience, as I’m sure it was for everyone - video recording, answering questions in chats, etc. I really enjoyed the talks, and I really liked how well all the sections, workshops, etc. were organized. I can only imagine the amount of work which had been done to make it happen in this difficult and stressful time.
I presented one of my research projects on novel plant-insect interactions. In this project, we explored variation in plant responses to grasshopper herbivory among the cultivars of the introduced Miscanthus sinensis.
Below is a video recording of my talk, and here are my slides.
April 11, 2020
Developed an undergraduate course in Invasion Ecology
I’ve finished developing the main outline of my course in Invasion Ecology which I hope to teach some day. This is a semester-long course; I see it as an upper-division undergraduate course but I think it can be easily adapted for a low-division undergraduate course or an elective graduate course.
Invasion ecology is a fascinating area to me: Why do some introduced species become invasive? Why do some introduced species fail to establish? What can we do to learn more about invasive species?, etc. All these questions have been driving my research since my doctoral studies at the University of Cincinnati. This course has been my ‘pet’ project since then, and I developed it based on both my own research and teaching experience.
I’ve designed this course to include weekly lectures and laboratories, weekly homework, online quizzes, as well as a mid-term and cumulative final exam. During both lectures and laboratories students would design and conduct experiments, develop and present group projects, participate in short local field trips, write article critiques, analyze case studies, and develop risk assessment protocols. I’d like my future students to gain all this experience and see themselves as scientists in training during that semester.
The course outline with examples of lecture slides, assignments and worksheets are available here. I’m currently exploring online alternatives for experimental laboratories and field trips. I really enjoyed developing this course, and I know I’ll enjoy even more teaching it some day!
Invasive spotted lanternfly, Lycorma delicatula, my current primary research object. We know so little about this unusual and one of the most aggressive forest insect pests in the eastern US. (4th nymphal instar, 7/17/2018, Berks County, PA)
April 1, 2020
Our paper on molecular gut content analysis of the invasive spotted lanternfly published in Insects!
So happy to see our paper on molecular gut content analysis of the invasive spotted lanternfly, Lycorma delicatula, published today in Insects special issue “Molecular Gut Content Analysis: Deciphering Trophic Interactions of Insects”! This paper summarizes the results of my work on molecular confirmation of diet of the spotted lanternfly. In this study we showed, for the first time, that host plant DNA can be identified within the gut contents of the spotted lanternfly and demonstrated the use of this approach for deciphering the host plant range of the lanternfly. I’m very happy to get it done and be able to share. I’m also very thankful to Darsy, Jessica, and Nurani - my mentees and great helpers - who helped me with routine DNA extraction, sequence editing, and depositing sequences to NCBI GenBank.
Here is our paper:
Avanesyan, A., and W.O. Lamp. (2020) Use of molecular gut content analysis to decipher the range of food plants of the invasive spotted lanternfly, Lycorma delicatula. Insects: Special Issue “ Molecular Gut Content Analysis: Deciphering Trophic Interactions of Insects”, 11(4), 215, doi.org/10.3390/insects11040215
A few selected figures:



March 18, 2020
Joined the Reviewer Board for journal 'Insects'
I have accepted an invitation to join the Reviewer Board for journal “Insects”! I’ll be serving as a reviewer in the following subject areas: molecular gut content analysis, eDNA, DNA barcoding, invasion ecology, plant-insect interactions, insect morphology, and host plant usage.
I’m happy to continue my longtime collaboration with journal “Insects” as an author, reviewer, guest editor, and now a member of the journal’s Reviewer Board. I’ve been enjoying all of my interactions with the editorial board of this journal and I’m always impressed by its timely responses, reviews, interesting special issues, and a speedy publication process!

January 3, 2020
I was very pleased to know that our grant proposal entitled “Identification of host plant use by the invasive spotted lanternfly (Lycorma delicatula) using next-gen DNA sequencing technology” has been funded by Maryland Agricultural Experiment Station Competitive Grant Program!
For this one-year study, we have proposed using a next-generation sequencing technology (‘Amplicon-EZ’) for meta-barcoding plant species from the gut contents of the invasive spotted lanternfly, Lycorma delicatula. Our current findings of plant DNA detection from the gut contents of the lanternfly nymphs using Sanger sequencing (Avanesyan and Lamp, manuscript is in preparation) suggest that while the observed lanternfly nymphs actively move on a host plant they may not utilize it for feeding. A substantial number of the nymphs (~90%) we have analyzed showed the ingested DNA from a host plant other than the plant from which the nymphs were collected. However, Sanger sequncing allowed us to detect and identify only DNA from one unique ingested plant. Conducting meta-barcoding of the lanternfly gut contents will allow us to detect multiple plant DNA fragments and detangle all possible associations with the host plants.


To date, we have successfully sequenced the gut content from one lanternfly nymph which yielded 139 ingested plant sequences. Among these fragments 12% were readable (i.e. long enough to use the BLAST engine in GenBank and identify the species), and among the readable ones - 12% were from the Bartramiaceae family, and 88% were from the Lamiaceae family. A substantial number of these plants are non-woody species which is very interesting (and important!) as the spotted lanternfly is considered to be a predominant tree pest. Looking forward to our work on this grant and more sequencing!!
December 26, 2019
Lanternfly paper published in PLOS ONE!
One year of work, four months in review, two months in revision, and here you go - a wonderful Christmas present from PLOS ONE! This paper summarizes the results of my morphological work on the spotted lanternfly, an aggressive invasive insect in the eastern US. In part, thanks to intensive training in scanning electron microscopy at the Laboratory for Biological Ultrastructure. In this study, I was able to investigate the external structures of the lanternfly mouthparts and tarsal parts, as well as a number of sensilla, which play an important role in the lanternfly primary contact with the surface of a host plant.
Here is our paper:
Avanesyan, A., Maugel T.K., and W. Lamp (2019) External morphology and developmental changes of tarsal tips and mouthparts of the invasive spotted lanternfly, Lycorma delicatula. PLOS ONE, doi.org/10.1371/journal.pone.0226995
A few selected figures:




It has been challenging and very interesting work. In this study, we focused on assessing changes in morphology of (a) the lanternfly mouthparts (stylets and labium), and (b) the lanternfly tarsal tips (arolia and tarsal claws) at each developmental stage. Our study revealed several interesting developmental patterns which potentially allow L. delicatula to better attach to a host plant and deeper penetrate to the host plant tissues at the late nymphal stages and adult stage. Here they are:
This is the first study on variation in the lanternfly external morphology across various developmental stages, and our findings are critical for investigating and predicting the lanternfly host range, and the lanternfly dispersal to new host trees at each developmental stage.
September 26, 2019
Bryan continues studying Molecular Biology at the University of Maryland!
I was very pleased to learn that my former mentee, Bryan, a high school student, was admitted to the University of Maryland where he continues studying Molecular Biology. Bryan was working with us during last Spring semester, and I was really impressed by his incredible progress during those several months. Bryan joined us in January knowing nothing about wet lab techinques for DNA barcoding process; he worked once a week only learning all the steps; and by the end of April he had successfully extracted genomic DNA from 100+ leafhopper samples, amplified plant DNA from leafhopper guts and succesfully obtained and deposited four plant sequences.
He is the first author on four GenBank submissions which were approved and are currently available in the NCBI GenBank database:
His work was the essential part of our paper on the use of molecular markers for plant DNA detection from leafhopper gut contents (the manuscript is currently submitted).
Bryan was also featured in The John Carroll School News.
I’m so happy for him!
September 16, 2019
Guest Editor for Insects, special issue on molecular gut content analysis!
I’m serving as a Guest Editor for journal Insects, Special Issue “Molecular Gut Content Analysis: Deciphering Trophic Interactions of Insects”. I’m co-editing this special issue with Dr. Bill Lamp. Our proposal for this special issue has been approved, and the special issue has just been announced on the journal’s website.
This Special Issue welcomes recent research on plant and animal DNA detection in the gut contents of insect herbivores and predators, respectively, as well as insect DNA detection in the gut contents of their predators, vertebrate or invertebrate. DNA-based protocols, short experimental reports, and original research articles, as well as review papers that broadly cover issues relating to molecular gut content analysis of various insect species (leaf-chewing insects, sap-feeders, soil-dwelling insects, etc.) and insect predators (both invertebrates and vertebrates) are of interest for this Special Issue. Studies that utilize both basic and advanced molecular biology methods are invited for submission.
The deadline for the submission is March 15, 2020. I’m planning to contribute to this special issue with some results of my DNA barcoding work, and I’m looking forward to receiving new submissions!
September 15, 2019
Presented at the 2nd UMD Postdoctoral Research Symposium
Last Friday, I had a poster presentation at our 2nd UMD Postdoctoral Research Symposium. I presented ‘the morphological part’ of my lanternfly research – the adaptations of the lanternfly mouthparts and tarsal tips to their host plant usage. I enjoyed both the symposium and the opportunity to present my research. This is the 2nd postdoctoral symposium at the University of Maryland, and I really liked its workshops and the opportunity to meet and talk to other postdoctoral researchers across the university.
Here is my poster.

June 10, 2019
Successful amplification of COI partial gene from dragonfly prey
Molecular identification of prey in predator diet - this is a new challenge in my DNA barcoding work; I amplify DNA from dragonfly prey items (a new lab project I’m involved in). In addition to a gut content analysis, I’m working with degraded DNA from dragonfly feces. Last week I successfully amplified and sequenced COI partial gene from dragonfly feces produced during the first 2 hours post ingestion. The sequence analysis with subsequent BLAST results showed that the prey item was a crane fly. Surprisingly enough the DNA from the crane fly wasn’t degraded and an isolated fragment of 667 bp showed good sequence quality. These exciting results can make identification of dragonfly prey possible and accurately confirm (or even poitn to new) trophic interactions in dragonfly natural habitats.

May 6, 2019
Guest lecture on invasive species tomorrow!
I’m very excited about my upcoming guest lecture tomorrow. This lecture is on invasive species for an undegraduate honors class on insect biodiversity. I gave the similar lecture last year but this year I have considerably modified the lecture content and class activities, I hope the students will enjoy it. I am also looking forward to sharing my latest results from my lanternfly project and showing the preserved samples.
Here is my lecture, and here is the worksheet for the students to follow our class activities.

April 27, 2019
We had a great time on campus enjoying yet another Maryland Day! While I was busy in our “Discover a Swamp” room, the rest of my family enjoyed ice-cream, baloons, trucks, books, and of course, our bugs!












April 26, 2019
Nina succesfully presented at her school research symposium
Last Friday, I enjoyed attending Eleanor Roosevelt High School Annual Research Symposium. Nina, one of my mentees, presented final results of her DNA barcoding work. This was her year-long project on identification of wetland and stream species of isopods using DNA barcoding. The overall idea of the project was to show whether wetland and stream communities demonstrate biological connectivity.
Nina presented a poster showing all the steps of the DNA barcoding process she learned during her year-long internship, as well as our current results. Using phylogenetic reconstructions, Nina has pinpointed three unique stream species and two unique wetland species which do not overlap.
April 15, 2019
Teaching summer course in Evolutionary Biology
I am super excited to teach a graduate course in Evolutionary Biology this summer as an instructor of record. This is a fully online course offered through the Master of Chemical and Life Sciences program (a content-based, professional master’s degree for middle and high school teachers, ranked #6 in the nation as best online master’s in biology programs). This is going to be a very interesting experience for me: I taught Evolutionary Biology as part of other courses before, but I have never taught it as a separate course. Also, this is my first time teaching online, and I am sure I will learn a lot!
The course has been offered for several years, so it has all the course materials set up. It includes various interactive assignments and discussions, as well as a final project which is a real lesson the teachers will demonstrate and will ‘take’ with them to their class. I really like this final part and that the teachers can create something new based on what they have learned, and they can apply it right away in their daily work. I am very much looking forward to teaching this summer course and meeting my new students!
March 13, 2019
Presented at the EB-ESA meeting in Blacksburg!
This week I presented at the Eastern Branch meeting of the Entomological Society of America in Blacksburg, VA. The meeting was very interesting, especially its special symposium on the spotted lanternfly. I enjoyed the talks, and I also was glad to participate in the poster session where I presented my poster with my current results on the lanternfly external morphology using scanning electron microscopy. I got interesting questions, several discussions, and a lot of valuable ideas. I can’t wait to start working on the paper based on our results. Bill and I also organized a symposium on novel plant-insect interactions where I presented all of my projects on grasshoppers feeding preferences on novel host plants (here is my presentation). The symposium went very well, I really enjoyed all the talks and I’m very grateful to all of our speakers for taking time to present and for basically making this symposium possible. Feels really great!


March 6, 2019
Scanning Electron Microscopy: Round 2
I’ve finished the 2nd round of scanning electron microscopy of the lanternfly mouthparts and tarsi which I conducted in The Laboratory for Biological Ultrastructure (my special thanks to Tim Maugel for his continuous help!!). We received beautiful SEM images, most of them are included now in my poster for the upcoming EB-ESA meeting. These images helped us to explore how different structures of the lanternfly mouthparts and tarsi change during the lanternfly development, and I’m very excited to start working on our new paper on the external morphology of the spotted lanternfly in relation to its host plants.
In this study we specifically focused on the structures which are responsible for primary contact of the spotted lanternfly with host tree surface (i.e. stylets, labium, tarsal claws, and arolia). We observed several interesting patterns:
Mouthparts - oval prominences and longitudinal striations on the mandibular stylets are more defined in 4th instar and adults; labium is more segmented in adults; numerous sensilla, including the bristle-like sensilla, on the tip of the labium are present at each developmental stage.
Tarsal tips - tarsal claws are more spread out in adults; arolia are fully developed at each stage; arolia surface becomes more wrinkled in adults.
We think that observed patterns are adaptations to host plant usage and correspond with a type of host plants utilized at each developmental stage, and we would like to focus on morphometric comparisons of mouthparts and tarsi next. Can’t wait for these new results!









February 23, 2019
Presented at the MOFFA meeting
Last Saturday I presented at the Annual Meeting of the Maryland Organic Food & Farming Association (Maryland Dept. of Agriculture, Annapolis, MD). It was a very interesting experience: it was my first formal talk on the spotted lanternfly and its host usage. The spotted lanternfly has not officially invaded Maryland yet (and we hope it will not - only one male individual has been found so far), but it was very important to share all we know about the lanternfly life cycle and behavior with our farmers and growers. Here is my talk.
January 5, 2019
This paper is on interactions of exotic leafhopper, Sophonia orientalis, with its novel hosts in Hawaii. It took quite a bit to analyze two-year data, to write a manuscript, and to wait for all of the reviewers’ comments. But now it is published in Environmental Entomology! Here is our paper, and here are we in our departmental news. Feels great!
November 30, 2018
I gave my first talk at our weekly departmental seminar: “Novel plant-insect associations: implication of the lack of coevolution”. It was very exciting and interesting experience, and I got many interesting ideas from the audience on my future research directions. My talk is here. Also, my labmates wrote a very cool seminar blog on my talk.
November 15, 2018
Busy 2018 ESA meeting in Vancouver!
This time the ESA meeting (2018 ESA, ESC, and ESBC Joint Annual Meeting) was especially busy for me. I served as a moderator in one session, a judge in two other sessions; I was responsible for setting up our UMD booth in the Exhibit Hall; I also gave a talk and presented a poster – whew! I spent a wonderful time listening various talks, discussing our work on the spotted lanternfly with our colleagues from Penn State University, and of course, enjoying beautiful Vancouver!
October 11, 2018
My systematic review and meta-analysis on feeding preferences of acridid grasshoppers on native vs. introduced plants has just been published in Plants, Special Issue “Plants Interacting with other Organisms: Insects”. It has been quite a long and challenging process with 3 rounds of revision and incorporating comments from 5(!) reviewers, but I’m really excited to see it published.

This systematic review entitled “Should I eat or should I go? Acridid grasshoppers and their novel host plants: potential for biotic resistance” aimed to identify patterns of grasshopper feeding preferences for native versus introduced plants and, consequently, a potential of grasshoppers to provide biotic resistance of native communities. I’ve worked on this review for a total of almost 2 years: I collected 2146 studies (from six databases) published during 1967–2017. Only 13 studies satisfied all of my inclusion criteria; from these, I was able to extract 63 records of feeding preference trials for 28 North-American grasshopper species. These trials involved direct comparison between consumed native and introduced plant material, and these records were included in the analysis.
I’m going to present a poster on this paper at the upcoming ESA meeting in Vancouver, CA. So I discussed with my PI the best way of presenting it since it is virtually impossible to fit all the details and analysis in one poster. His advice was very simple: to present the main message I’d like to tell people and support it with evidence. I was thinking about this one message, and I came up with five(!) things which were the most surprising for me and which I definitely would want to share:
I collected these data and wrote the main parts of these manuscript while we lived in Iowa. So the graphical abstract (see above) is special for me: it is a shot of a grasshopper eating our palm tree on our balcony in Iowa, on the 3rd floor!
September 8, 2018
Summer is over. It was a busy Summer - full of interesting work, trips, and a lot of writing. Looking back, I’m happy to share a few highlights.
A couple of months ago I’ve been offered to serve as a Subject Editor for the Journal of Orthoptera Research. It was super exciting! I served as a reviewer for this journal before for about 2 years, and I really enjoyed it. I also constantly refer to this journal in my research since it is the only journal we have so far where you can find everything about grasshoppers in one place! My editing work is in a full mode now - I’ve been editing the manuscripts in the areas of Biodiversity & Conservation, General Ecology, and Molecular Biology.
I was also very happy to be featured in our departmental newsletter!
During the past summer, I served on the planning committee for the first (ever!) UMD Postdoctoral Research Symposium. The symposium is almost here (on Sep 17), we’ve planned several interesting workshops (getting grants, designing a new course, etc.) - should be fun!
I’m very happy to be back to the PlantingScience program, this time as a member of the Master Plant Science Team! I will serve as a liaison for one class of high school students and as a scientist mentor for 2-3 teams. I had incredibly rewarding experience participating in the Digging Deeper project last year and I’ve decided to stay with PlantingScience forever! I’m very much looking forward to continuing my work with teachers and students this academic year and together with them enjoy science!
And last but not least was that one of our two lanternfly proposals has been funded by The Maryland Agricultural Experiment Station, McIntire Stennis Forestry Research Program. These news came as a wonderful surprise on Friday, Aug 31 late afternoon (the last possible day for such notifications) with the starting date Sep 1. This is super exciting, my work on the spotted lanternfly began a few months ago, now we have some preserved specimens, and I don’t have to wait anymore to ‘officially’ start exploring them. More details to come!

May 7, 2018
We had a wonderful time on campus celebrating our annual Maryland Day! I was really impressed how well everything was organized and how many things there were to do with friends and family, and of course with little and not so little kids. We came there for 2 hours and stayed for the whole day! My daughter enjoyed our insect petting zoo very much, although she refused to touch the tarantula (well, I don’t blame her); but “scooping bugs” was by far her favorite thing to do. She also was fascinated with all the insect models and stickers with insects; and of course she immediately “borrowed” the toy-grasshopper from my office desk once she had spotted it.
We tried some other things, too: we got to the barn where we touched sheep and watched beautiful horses. Ice-cream, a balloon, a police cap, lots of candies, and wonderful weather were included! This was the first time I took my daughter to my work - she said she liked it very much, and I’m pretty sure she thinks now that my work is Maryland Day every day!












April 20, 2018
My first adventures in the aquatic world
Being almost exclusively ‘terrestrial’ person in my research it has been quite an interesting experience for me to “get wet” with both Dr. Lamp’s lab and his Aquatic Entomology class. Although I am a TA in this class I fully participate in all the student activities. It’s fun, but also I can’t stop being surprised of how different things are under water. Well, here are my first impression:
The first surprise for me was the fact that apparently there is no such thing as “traditional” plant-insect interactions in aquatic communities. Everyone (all insects) eats almost everything (all leaves which fall in a stream), and all “tastes” the same as long as it provides enough nutrients.
I also didn’t know that it is not a leaf itself that attracts aquatic insects, but a biofilm - a bunch of different microorganisms which stick to each other and to a surface of a leaf. I learned that “the leaf with such biofilm for aquatic insects is like a toast with peanut butter for us: a toast itself is edible of course, but with peanut butter it is a completely different food” (borrowed from Becca W.)
Below are some pictures from my first collections and insect identifications.




April 19, 2018
Exploring family-friendly places in Maryland
If not every weekend, but at least every other weekend we try to get out to a new place with our growing little one. I was glad to find out that here in Maryland we could find plenty of such opportunities within an hour-drive from home, and often less than that. Here are the places we have visited so far. Our daughter was super excited about each of them and if she could she would go to each of them every day:
Feb, 2018 – it was cold, windy, and rainy, but we made it! We’ve picked a nice kids-friendly trail with a gorgeous view on the Potomac River.


March, 2018 – it is a nice open air zoo with 20-min safari bus ride. We fed goats and llamas, enjoyed birds, giraffes, camels, cheetahs, and tried not to wake kangaroos up.




April, 2018 – this is exclusively for kids, of course. It has several themed rooms – our daughter enjoyed playing with water, construction tools, blocks, pretend snakes in the pretend rainforest, and even a little theatre. Finally we all enjoyed playing outside on a very original playground with a castle and a hidden slide which for some reason reminded me of the slopes in Colorado.




April, 2018 – we apparently went not to the best trail for kids, but according to what I’ve read the park has a lot of different trails with beautiful views. So we are looking forward to exploring it more. Nevertheless, the weather was beautiful, and we were glad to spend a couple of hours hiking and exploring all the sticks and pine cones our daughter could find (extremely hard task). I was also on watch out for the spotted lanternfly, but nothing so far. Not yet, I guess..



January 20, 2018
Starting a postdoc at the University of Maryland
I’ve just started as a Postdoctoral Associate at the Department of Entomology at the University of Maryland! This job offer came to me as a wonderful Christmas present, and we packed and moved to Maryland within 2 weeks.
I am working in Dr. Bill Lamp’s lab, where I will continue my research on ecology and evolution of plant-insect interactions. My primary focus will be novel associations between native and introduced species and their effect on natural and managed systems. Dr. Lamp has been my mentor for a long time, and we’ve been collaborating on several projects since my doctoral studies in Cincinnati. So working in his lab as a postdoc is a great opportunity for me.
I’m very excited about my new position, we love our new place in Maryland, and I am looking forward to my next couple of years in the Lamp lab!
October 20, 2017
Week 5 of Planting Science program
It has been 5 weeks of Planting Science program and my online mentorship of high school students teams in their plant investigations.
I really enjoy it and I can't stop being impressed how well everything is organized: all possible questions I might have are in weekly newsletters, mentors' online resources, or in group announcements. And if (if!) I can't find an answer to my question I can always post it on our forums and will get help right away – literally, within 10-15 min. Amazing!
I've been mentoring three research teams so far and I am serving as a liaison for a teacher and her three student teams. Interactions with students are incredibly rewarding, and I feel very lucky to be involved in this program!
October 4, 2017
New (or old?) mode of transport for invasive species
<!–<p class="ic4f-blog-list-excerpt">Carlton et al. Science 29 Sep 2017:
”..We document 289 living Japanese coastal marine species from 16 phyla transported over 6 years on objects that traveled thousands of kilometers across the Pacific Ocean –>
Carlton et al. Science 29 Sep 2017:
”..We document 289 living Japanese coastal marine species from 16 phyla transported over 6 years on objects that traveled thousands of kilometers across the Pacific Ocean to the shores of North America and Hawai‘i. Most of this dispersal occurred on nonbiodegradable objects, resulting in the longest documented transoceanic survival and dispersal of coastal species by rafting…”
The more I study invasive species the more I realize how little I (we?) know about them: they can adapt to a new habitat relatively easily, they successfully compete with native species, they disperse fast, and apparently long distance is also not an obstacle for them!
July 29, 2017
Last week I spent in Colorado Springs at the Digging Deeper Summer Professional Development workshop which is part of my Planting Science fellowship I received earlier this year. It was wonderful experience: I enjoyed meeting biology teachers and other scientist mentors, and I’ve learned a lot. If you would have asked me “Where does the tree mass come from?” a week ago I would have hard time explaining this!
We also have a wonderful trip to the Garden of the Gods: red rocks are just gorgeous. Every time I go to Colorado I discover something new. We also went to a “plant walk”, which was a 40 min-walk from the BSCS building to our hotel accompanied by identification of plants we saw and amazing stories about them – many thanks to our ‘tour guide’, Catrina!
I’m looking forward to start working with teachers and their student teams this Fall!

May 10, 2017
Our GenBank submission has just been approved!
Our GenBank submission has just been approved! Here it is:
Backer, S., Christiansen, K., Christofferson, D., Geisinger, S., Graving, S., Jones, K., Miller, R., Omanovic, E., Piatt, D., Reyes-Zuniga, K., Salazar-Klock, L., Sopher, K., Welsch, A., Avanesyan, A., and I. Hazan. (2017) Salvia rosmarinus isolate rs GAPC-2 gene, partial cds. Direct Submission, GenBank Accession no. MF074139
Many thanks to my students who did a great job choosing a plant species (in the first place!) for which a partial sequence for the GAPDH gene (GAPC-2, 1139 bp) previously hasn’t been described, and who, of course, worked hard on isolating and cloning this part of the gene.
April 21, 2017
Three poster presentations of my students. Great job, guys!
Today my students presented their molecular biology term projects at 4th Annual Grand View Scholarship Symposium. Very exciting! We were very nervous at the beginning of the semester when we started cloning the GAPDH gene from cilantro and rosemary (my students’ plant choice!) It was a very challenging project: sometime a time period of a lab was simply not enough to finish PCR or check bacterial growth. Students did a good job coming to the lab after or between classes to take care of their samples. Many thanks to our lab administrator, Jennifer Donnelly, and the department head, Idit Hazan, for their help with preparing samples and taking care of plants, as well as all the supplies we needed during the semester.
Anything could have gone wrong at any time, but here we go, we have three beautiful posters and 13 happy students who are proud of their work:
Furthermore, sequence of the GAPDH gene from rosemary, turned out to be particularly good, and we are currently preparing it for submission to NCBI GenBank. Feels great!

March 10, 2017
My paper from my postdoctoral work at the University of Wisconsin-Madison has been just published in Insects, in special issue “Invasive insect species:
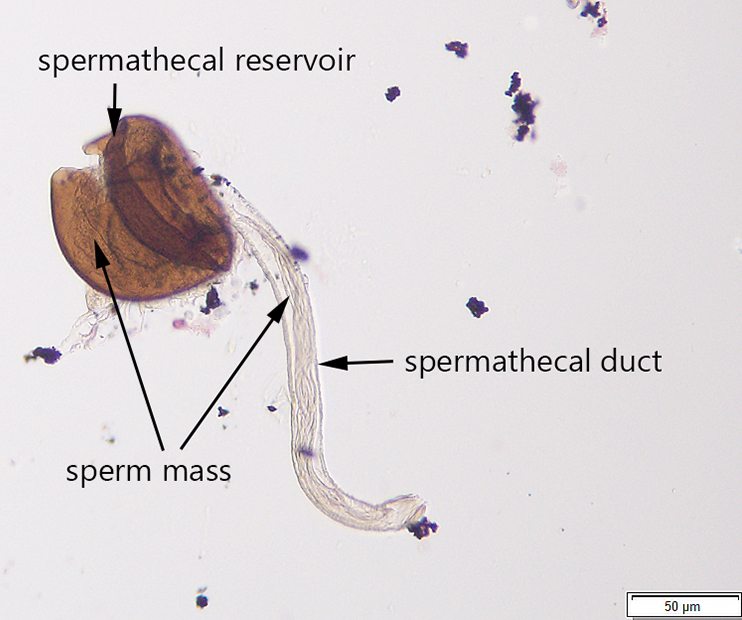
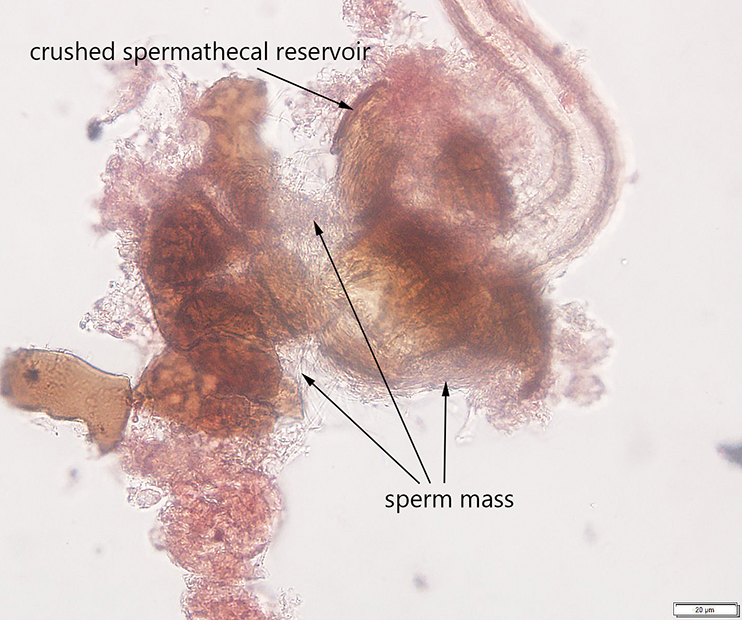
Avanesyan, A., Jaffe, B.D., and C. Guédot (2017) Isolating spermatheca and determining mating status of the invasive spotted wing drosophila, Drosophila suzukii: a protocol for tissue dissection and its applications. Insects: Special issue “Invasive Insect Species”. 8(1), 32; doi:10.3390/insects8010032. Invited paper.
This paper describes a step-by-step protocol for tissue dissection, isolating spermathecae, and determining the mating status of females which I developed specifically for invasive Drosophila suzukii.
Many thanks to Claire Mattmiller, a high school student whom I mentored, for numerous fly dissections she made and several illustrations which have been included in this manuscript.
December 16, 2016
My last dissertation chapter is published! Now I can retire...
It will be included in January issue of The Journal of the Torrey Botanical Society:
Avanesyan, A., and T.M. Culley (2017) Tolerance of native and exotic prairie grasses to herbivory by Melanoplus grasshoppers: application of a non-destructive method for estimating plant biomass changes as a response to herbivory. The Journal of the Torrey Botanical Society 144(1):15-25.
That was one of my last experiments on plant responses to grasshopper herbivory which I conducted in Ohio and Maryland. In this study we wanted to estimate changes in plant biomass (a proxy for plant tolerance) without clipping the plants. Interestingly, we’ve found that for the most grasses we used the predictor variable [plant height x number of leaves] was the best. Most likely it works for grasses only, but I am curious to see what combination of measurements would work for other plants. Maybe I will test it in some future experiments..
August 28, 2016
Started working at Grand View University
I am very excited to join Department of Biology at Grand View University in Des Moines, IA as an adjunct Genetics Instructor! I’ll be teaching two sections of an upper-level Genetics laboratory course for biotechnology majors this Fall, and (hopefully!) Molecular Biology lab in the Spring.
The university is quite a commute (2 hours from my home, oh well..), but I am really excited about teaching genetics – one of my favorite courses and research areas. The lab equipment and supplies are really impressive, small classes, cozy buildings, friendly faculty – I’m very much looking forward to starting my semester!

March 2, 2014
Another story about my research on plant DNA detection from grasshopper gut contents has been just published in India!
The story is called “Gut Instinct”. This is a science magazine, Down to Earth, published by the Society for Environmental Communications, India.
February 8, 2014
My protocol paper just came out in the February issue of Applications in Plant Sciences!
The press release for this paper is here.
The paper was also featured in several places such as ScienceDaily, ScienceNewsLine, and Bio-Medicine.
December 28, 2013
Plant DNA detection from grasshopper gut contents... published!
My paper “Plant DNA detection from grasshopper gut contents: a step-by-step protocol, from tissues preparation to obtaining plant DNA sequences” has just been accepted for publication in Applications in Plant Sciences!
It will come out in February 2014. This paper describes a protocol for isolating plant food DNA from insect guts, developed specifically for grasshoppers. It is a very convenient and relatively quick PCR-based method which allows accurate confirmation of food consumption by insect herbivores. Given that grasshoppers are important agricultural pests and dominate insect herbivores in the grasslands, knowledge of their feeding preferences is critical for both crop management and landscape restoration.
The paper includes this video which I made to illustrate the main steps of dissecting a grasshopper.
I am currently applying this protocol to exploring grasshoppers’ feeding choices with regard to native and exotic plant species, which is another application of this method – to help managers to effectively control invasive plants and/or predict invasion.
November 28, 2013
First place at ESA meeting in Austin, TX
Last week I presented my research project on molecular confirmation of insect diet at the Annual Meeting of Entomological Society of America in Austin, TX (November, 2013) and won the first place in the Graduate Student Ten-Minute Paper Competition in the plant-insect interaction section!
Here’s my presentation: Plant DNA detection from grasshoppers’ gut contents: method and applications.
March 20, 2013
Second place at ESA Eastern Branch
Yesterday I presented at the Annual Meeting of the Eastern Branch of the Entomological Society of America and won second place in the PhD student oral competition! There were 13 entries representing Penn State, Rutgers, Texas A&M, West Virginia, Cornell, Maryland, Tufts, Virginia Polytechnic Institute, Northeast Forestry University (China), and Carleton University (Canada).
Here’s my presentation: Feeding preferences of the generalist insect herbivore, Melanoplus femurrubrum grasshopper, on invasive and native plants.

January 8, 2013
Last summer I started my grasses/grasshoppers feeding experiments in the field. The main problem I had to solve was building the cages.
The cages had to be high enough to accommodate the plants. They also had to be low enough so that I wouldn’t lose my grasshoppers there. I also needed some sort of a separator to divide the cage into two halves – for control plants and plants with grasshoppers. From literature, I knew that the cage should be metal, so that grasshoppers won’t chew through it and escape. Also, there should be screen walls – to let enough light into the cage (both for plants and grasshoppers).
After some searching I ended up using open air aluminum screen cages (16×16×20″ Repti Breeze Aluminum Screen Cage, Zoo Med Laboratories, Inc., California, USA). These cages are meant for keeping reptiles, but their material, size, and shape were exactly what I needed.
First I removed the bottom of the cage (it is just a plastic plate).


For a separator I used a small regular window screen – you can find those in Lowe’s or Home Depot, they are cheap. You can assemble your own, but I found one that was the perfect size for my cages. On both sides of the separator I attached a thick window sealant strip – so that it fit tightly against the screen walls of the cage and did not leave any holes for grasshoppers to move across the cage and feed on control plants – and ruin my data.

I placed some extra soil inside the cages to cover all possible holes at the bottom. It is a good idea to add even more soil than is needed: if it rains part of the soil will get washed out, uncovering new holes. I also added more soil outside along the edges of the cages.

I started my experiments by placing two different grass species in each half of the cage, but this year I will be planting four different grasses – I expect them to fit (UPDATE: they did fit perfectly!) I would not go with more than four though…

These are field experiments – so fixing cages to the ground is another problem. I used two ropes and four metal hooks; this proved to be very stable, even during heavy rain and strong wind.

The cages turned out to be very convenient not only for field experiments but also for a temporarily keeping grasshoppers in the lab (I usually keep them in the greenhouse). The cages have two doors, and the lowest one is designed specifically for cleaning the cage without disturbing the reptiles – that works for grasshoppers, too! I also often use this door for placing new grasshoppers in the cage; they tend to jump up when released in the cage from a collecting bag and this way they will not escape.
I hope this might be useful to someone who conducts similar experiments.

January 7, 2013
The board is your friend! (part 2)
I liked the idea of writing a lab flow on the board so much, that soon I started to add “Next lab assignment” rubric as well. As I usually need all space on the board during the lab, I’ve decided to write a homework assignment for the next lab in the right top corner of the board. I never need that space, whereas my students always know where to find the assignment.

I started writing the assignment soon after a few students asked me several times about the homework. It was an unexpected question for me; usually all assignments are posted on the course webpage and are listed in the student’s lab manual along with all deadlines. I thought maybe it would be helpful if I would remind them every time what they should focus on for their homework: things such as, for example, reading chapter 3, pre-lab for week 2, bring your computers/textbooks etc.
Again, I’ve found this to be helpful for students: they can ask more specific questions about the homework before they actually start doing it at home. Also, I’ve noticed that a few students from time to time started doing their homework in class. It was the students who finished the lab earlier than the rest of the class and didn’t want to waste the remaining time.
It made me think that they considered the “next lab” note as a continuation of the lab flow that I write on the board. On one hand, it is a good connection between the homework and material covered in class: the students can start doing the homework while they still remember the lab and have an opportunity to discuss it with their classmates and me. On the other hand, I am a little bit concerned about the quality of their work when they are working so fast. I am still thinking.
As for my teaching side, writing the homework assignment helped me emphasize things in the lab flow which students should focus on during the lab and which will help them later in their homework. This approach definitely decreased the amount of questions about the type of the assignments; instead we focus more on specific questions related to the homework’s content. In fact I have almost stopped worrying about how well my students understand their homework assignments, not to mention the decreasing of amount of emails with general questions. :-)
Also, I started thinking about what else I could put on the board to help me in class. Since I used a projector very often for different information, I couldn’t have any notes displayed on the screen for the duration of the lab. Also, I was worried that additional notes on the board might be unnecessary. So I started watching my explanations during the lab and marked (in my mind) the typical moments when I actually need to use the board.
It turned out that the most often I referred to the board when I needed a scheme explaining the order of steps of an experiment: liquid from what tube goes to what tube, what we add to the tube, etc. Another typical situation when I needed the board were group projects: dividing responsibilities between groups and group members. Since then each time I am dealing with a complex experiment or group work I use the remaining space to write what I think is important for the current lab. I’ve found that besides saving time and organizing the work, these notes also provide visual cues for students for understanding the guidelines in the lab manual, as well as seeing their own impact in any team project.
December 17, 2012
I teach biology labs. Our typical lab usually includes a short lecture, a discussion about homework (so called pre-labs), a wet lab (i.e., an experiment), and exercises or answering questions. Having so many activities I found myself trapped in the situation where I have to explain the next lab phase to students while some of them are still working and are not listening to me. Even those who had finished the previous lab phase were not paying attention to me because of the noise from the others. I didn’t feel comfortable at all: I was rather helpless and quite unimportant. Besides, my teaching is also complicated by my accent: I am afraid that even if my students hear me, they won’t be able to figure out what I’m trying to explain.
Such situations were not very comfortable in my past either – even when I taught in my native language. One simple solution for professors in my native country was to speak louder – eventually all students would start looking at her and listening to the lecture. We were used to it as students, we did it later as professors. There were, of course, different versions of this approach: to speak very loud, to pause the lecture, or, as yet another option, I remember one professor used to start whispering when students continued to talk. I tried some of these approaches, but I didn’t like any of them. I thought all of them were artificial ways to attract students’ attention to what the professor had to say.
Over the years, I started thinking that such situations simply will not occur if the professor naturally attracts students’ attention by doing and/or telling something interesting, as well as by keeping students (and herself!) busy and organized during the class.
Keeping this in mind I looked for ways to prevent these uncomfortable situations from happening, as well as ways to reduce the possibility of students misunderstanding me due to my accent. One day I followed the suggestion of one of my professors about a particularly complicated lab: she suggested writing the lab flow on the board. Which I did.

I told my students that when they had finished one part of the lab they could look at the board for what to do next. If I needed to give an explanation before each task, I waited until everyone started doing the task, I would then ask them to pause and listen to my brief explanation, and then they would continue. I’ve noticed that it helped me and students to be more organized; as a result, I had less pauses in class.
Writing the lab flow on the board for that complicated lab was helpful. In fact, it proved helpful for any lab. Since then I have been writing flows for each lab, even the simple ones with few activities. I have also realized that it is important to make my writing as clear and legible as possible and to refer to the lab flow on the board often; otherwise, students simply wouldn’t pay attention to what I wrote. The last bullet in the lab flow is especially important: it means the end of the class – at which point students know what they should turn in before they go, what needs to be cleaned up, etc.
Based on feedback from students, this lab flow idea is helpful for them as well. Thinking back to my past teaching experiences, I wish I knew this at that time. I am sure this simple approach would’ve helped me keep students’ attention and make their learning experience more enjoyable.
November 21, 2012
At the end of each semester I receive students evaluations. It was a very difficult moment for me when I had just started teaching in the US. Mostly because there is no such thing as students evaluations in my native country. It was one of many new things for me. I felt awful when I first saw “she struggled with explaining concepts”, or “she was quiet and I couldn’t hear her”, or “she graded hard”, or even better “she didn’t answer the questions and couldn’t conduct discussions”…
I know that it is not only me who feels uncomfortable reading a bad review from students. A professor I knew joked that she usually drinks a bottle of wine before sitting down to read her evaluations. Someone else told me that students always complain about professors and he didn’t want to know what they wrote about him. At a conference, I heard that some TAs simply stopped reading evaluations, while others never even opened them.
I understand such feelings. I also have things I am afraid of. I am afraid that students will not understand my accent; I am afraid I’ll have to ask them to repeat the question several times until I get it. Terrible feeling. I am afraid that I indeed will not know the answer and students will infer that I might not know other things as well. However, one of the things I am not afraid of is to open and to read student evaluations. I am even looking forward to them. Here is why.
First, people don’t want to read bad things in the reviews, but they forget that there are also good things there. There is always a question “what do you like in your professor/TA?” in any evaluation; and there are always some good things in the answer. Anyone, I think, would like to read something good about themselves. I know I would.
Second, reading evaluations is part of my job. It just needs to be done. Like grading, for example. It is my responsibility as a TA (and as a future professor) to read student evaluations and to use this information to improve my teaching.
And, finally, I think it is again a question of respect for my students and their time. My students spent time answering the questions in the survey and writing their reviews. I have to appreciate that.
November 17, 2012
<!–<p class="ic4f-blog-list-excerpt">In my very first evaluation that I received from my students here in the US I saw this comment: she is very straightforward.
I wasn’t sure what that meant. –>
In my very first evaluation that I received from my students here in the US I saw this comment: she is very straightforward.
I wasn’t sure what that meant. At first I thought it was just a language thing: I didn’t have a large vocabulary during my first year of doctoral study, so teaching was often difficult. Sometimes I wasn’t sure how to explain a concept, sometimes I didn’t know how to ask a question, or make a comment, or ask students to do this or that – using the right, appropriate words. These things seem simple enough, but for me they were not simple, especially during my first semester of teaching in a new country.
Very straightforward. Was I not polite enough? I certainly didn’t mean to. In my next semester I tried to use words like “could you please”, or “would you like” more often. However, another problem came about: now every time before asking students to do something I had to think about how to ask them. Since I was still working on improving my English (and still working!), that took some serious effort! But it seemed like I was solving the problem.
Today it is easier for me to talk to my students. I am much more comfortable answering their questions, helping them when they are wrong and encouraging them when they are right. I know I try. However, when it comes to giving them specific feedback on grading, my hard times return.
I thought about this over and over, trying to understand the roots of the problem. I think it cannot be just language.
Maybe it’s a culture thing? After all, I’m from another country. I recalled the time when I was a professor in my native country and realized that I never worried about how to communicate with my students. And here’s why: the important thing was the amount of knowledge the professor had. The amount of knowledge meant authority; how to share that knowledge with the students was secondary. And overall, the teaching process was more strict: students didn’t have much freedom; they didn’t change advisors, committees, research topics, or their majors very easily – if at all. That must have reflected on the way professors talked to students and how students talked – or, better, listened – to their professors.
But I think there’s something else – not just using the right words. After all, memorizing the words I should (or shouldn’t) use with students didn’t really help much. So here’s my “big idea”: I think the words reflect how I see myself, my status – comparing to how I see the status of my students, and what I think of them. Do I think that I know everything on a given topic much better than they will ever know; and does this give me a special right to talk to them from this position of superiority? Or do I think of them as future colleagues who just happen to be studying what I had studied earlier, and maybe later some of them will know this subject deeper than I do – after it becomes their research area? Essentially, it is a question of respect: the more I respect my students – the easier it is for me to talk to them. Even when I need to provide detailed feedback on harsh grading. Because I think that, for them, obtaining such feedback is very much like, for me, obtaining feedback from my professors on my own research: they always want me to change this or that, but I also know they want to help me and they want me to succeed. Right?
And thus, the problem of finding the right words slowly went away. But I still need to work on my vocabulary.
October 29, 2012
Professor Sprout: How this blog started
At first this blog was anonymous. It lived at www.professorsprout.me – an address which captured my attachment to biology and, yes, the Harry Potter books. Eventually I decided to move it to this new website that has a more formal name. But I’m keeping my first post unchanged – with all its Harry Potter references – for the sake of a little bit of magic.
I called my blog Professor Sprout because I teach biology, conduct research on plants, and love the Harry Potter books!
At Hogwarts, each year students learn something new. Each year they meet new teachers. Each year they are exposed to different teachers’ personalities and different teaching methods. Eventually, some of the students become teachers themselves – and return to Hogwarts to teach new students who arrive each year to start their own journey of discovery!
I love this stuff. I’ve lived in “the world of academia” for many years. In my native country overseas I’ve come a long way – from first-year student to university professor. A few years ago I’ve started a similar journey in the United States, which has become my new home. This time I am a doctoral student, once again studying to become a university professor. Although this time I feel different – not only because I am older or because I speak a different language. Having went through some twists and turns along the way, I have started to think much more about how I study, and how I teach, and what I can do to become a happy and successful professor here in America.
I have decided to start this blog in hopes that it will help me find a balance between my previous experience and the new knowledge I’m discovering. My blog will be about different things; things which have an effect on my teaching, my research, and the way I advance towards my degree. By sharing my thoughts through this blog, I hope to better understand what kind of professor I actually want to become during this new academic journey.
So let’s see where the Hogwarts Express takes me and what new stations I am about to discover!
